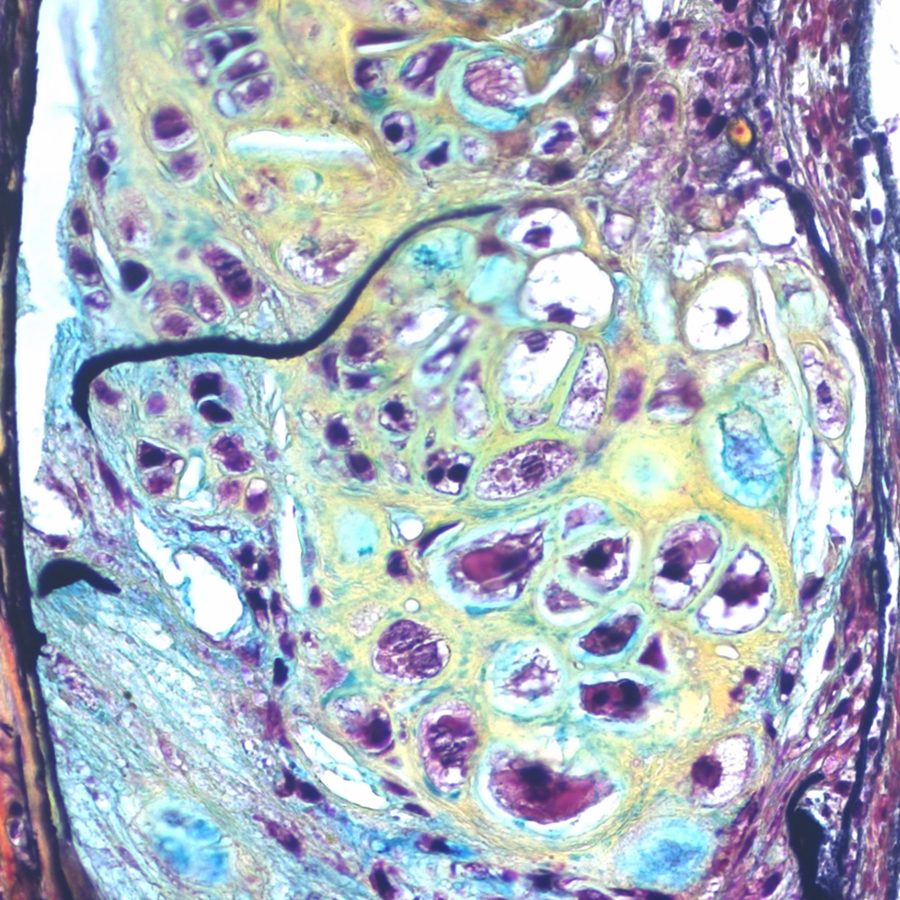
Scientists at Genuity Science’s Advanced Artificial Intelligence Research Laboratory and colleagues at Yale University School of Medicine today announce the discovery of a biological mechanism that causally underpins aortic aneurysm. They identify a specific disease-prone cluster of smooth muscle cells (SMCs) in the aortic wall and follow how, with the loss of the protective effects of transforming growth factor beta (TGFß) and increased expression of Krüppel-like factor 4 (KLF4), these SMCs are progressively reprogrammed into fat, bone, cartilage and other cell types. This process results in a weakening of the structural integrity of the aortic wall and leads directly to the development of aneurysm. They also demonstrate that disrupting this process can reduce the incidence of aneurysm and of atherosclerotic plaque burden, pointing to promising new drug targets for a deadly disease that is currently only treatable through surgical or endovascular intervention. The study is published today in the online edition of Cell Stem Cell.
This paper is the latest in a series of breakthroughs in the understanding of the causal biology of cardiovascular disease to emerge from the combination of the single-cell biology expertise of Professor Michael Simons and his lab at Yale with Genuity Science’s capabilities in DNA and RNA sequencing, and in artificial intelligence, led by Dr Tom Chittenden. In 2019, the group demonstrated the power of this approach for predicting cardiovascular phenotypes resulting from reprogramming of endothelial cells and then for slowing and reversing atherosclerosis. A remarkable feature of today’s study, contrasting with traditional studies of bulk tissue, is that the authors have constructed a mouse model of the disease and effectively watched its progression both through gene expression analysis and directly through imaging.
“This is single-cell science at its revolutionary best. Building on the Yale team’s precision model biology, our deep learning has pinpointed and then observed month by month how a group of cells drive this disease,” said Dr Tom Chittenden, Genuity Science Chief Data Science Officer and co-senior author on the paper. “This is an in vivo-validated starting point for drug discovery in a condition that kills tens of thousands of people every year. Importantly, it also represents a replicable approach we can apply to uncover the causal biology of just about any phenotype.”
“This is breakthrough science – with the potential to positively impact how we treat and prevent aortic aneurysm, at the same time underscoring the power of this new model of biomedical research,” said Dr Simons, Professor of Medicine and Cell Biology at Yale. “The demonstration of the existence of disease-prone cell populations is completely transformational with respect to our understanding of disease biology and ability to tailor disease- and patient-specific therapies. But identifying them requires not just deep single-cell biology know-how but also the ability to generate and analyze extraordinarily large and complex datasets. Working with Genuity Science has enabled us to do this, and I am increasingly convinced that our collaboration is going to inspire more people to adopt this approach.”